Longsee - COVID-19 Professional Rapid Test Kits
15,25 €*
Available, delivery time 4 - 7 Working days
Purchase Longsee Rapid Detection Kit for Quick Testing of 2019-nCOV Ag
Detect 2019-Ncov infections quickly and reliably with our Longsee rapid detection kit. Within 15 minutes, the kit provides reliable results. All necessary materials are included in the kit and no further equipment or laboratories are necessary. Thanks to a sensitivity of 95.51% and a specificity of 99.72%, professional application is guaranteed.
Features of Longsee Rapid Detection Kit for 2019-nCOV Ag
✓ Listed on the "Common List" - Device-ID: 1216 at the European Commission
✓ 95.51% sensitivity, 99.72% specificity
✓ Overall sensitivity (Paul Ehrlich Institute evaluation): 100%.
✓ Detects virus variants such as delta and omicron
✓ Only provided to authorized groups of people (professional test)
✓ Results in 15 minutes
✓ Only for in vitro diagnostics
✓ No equipment required
✓ CE certified and suitable for use in the German market
Our tests are all CE-certified and suitable for use in the European market.
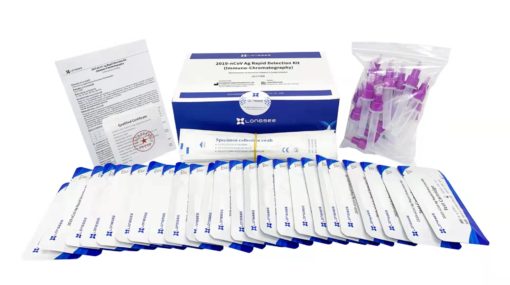
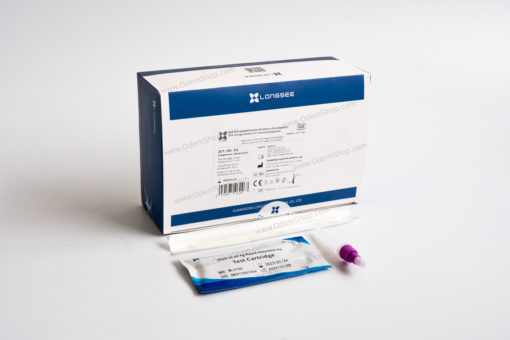
Contents of Longsee Rapid Detection Kit for COVID-19 Antigen Testing
25 sterile swabs
25 test cassettes
25 extraction tubes
1 instruction manual
Order now and detect COVID-19 quickly, reliably, and easily with the Longsee Rapid Detection Kit!
Why Order Longsee Rapid Detection Kit for 2019-nCOV Ag from OdemShop?
✓ MONEY-BACK GUARANTEE
Our goal is your satisfaction with your purchase. If the purchased goods do not meet your expectations, you can return them within the 14-day cancellation period, and you will be refunded the purchase price.
| Comparison - Professional COVID-19 Antigen Rapid Tests | |||||||
|---|---|---|---|---|---|---|---|
| Manufactured by | Hecin | Testsealabs | Bioteke | Longsee | Green Spring | Roche | Siemens |
| Sensitivity | 97.09% | 92.10% | 96.49% | 95.51% | 96.77% | 96.52% | 97.25% |
| Specificity | 99.78% | 98.10% | 99.28% | 99.72% | 100% | 99.60% | 100% |
| Manufactured in | China | China | China | China | China | South Korea | China |
| Listed for the "EU common list" |
Yes, in category A | Yes | Yes | Yes, in category A | Yes | Yes | Yes |
Frequently asked questions before you buy the Longsee Rapid Detection Kit for COVID-19
What is Guangdong Longsee Biomedical Co Inc?
Guangdong Longsee Biomedical Co. Inc. is a leading biomedical engineering company. With a strong research and development department and an extensive product range, the company offers solutions for a wide range of healthcare applications, e.g., manufacturing the Longsee Rapid Detection Kit for COVID-19 mentioned here.
What is a Longsee Rapid Detection Kit for COVID-19?
The Longsee SARS-CoV-2 test is a rapid test method for the rapid detection of infection with Covid-19. It is based on immunochromatography technology, requires only a few test steps and provides a result within 15 minutes.
Can the Longsee rapid test detect mutations?
Yes, mutations such as the Omikron mutation can be detected with the COVID-19 antigen rapid test from Longsee. This is also confirmed by the Common RAT List of the HSC (List of COVID-19 Antigen Rapid Tests of the European Commission).
Is the Longsee rapid test safe?
The Longsee COVID-19 Antigen Rapid Test has been evaluated by the PEI and has an overall sensitivity of 100%. The sensitivity of the test is 95.51% and the specificity is 99.72%. As the test is on the "Common List" (list of the European Commission), it can be assumed that it is classified as safe.
How does the Longsee Rapid Detection Kit for COVID-19 work?
By nasal, nasopharyngeal or oropharyngeal sampling.
How long does the test result of the Longsee Rapid Detection Kit for COVID-19 take?
15 Minutes.
How is the Longsee Rapid Detection Kit for COVID-19 packaged?
There are 25 tests per pack/box, in a whole box 700 pieces.
How long can professional Longsee Rapid Detection Kit for COVID-19 be stored?
Can be stored at room temperature or from 4 to 35 degrees for up to 12 months.
Does the Longsee test recognise the Omikron variant?
Yes, the tests have been checked by the Paul Ehrlich Institute. The Omikron variant is fully proven.
How did the tests perform in the Paul Ehrlich Institute's control list?
The tests are on the list that meet the sensitivity criterion. You can read the exact details here.
Who may use the tests?
These tests are intended for professional use only. You can find layman antigen tests here
Where can I find more information on antigen testing?
For comprehensive information on antigen testing, you can consult our Blog
Technical Data
Packaging
25 packs
Carton
1050 pieces
Standards - and Market Compliance
Professional use (for professional use only)
Quick test 3 in 1
Approved in Germany
BFarm Number: AT731 / 21
Listed for EU-wide recognition in the "Common List of the EU" of the European Commission - Directorate-General for Health and Food Safety
CE-certified and reimbursable test listed at the Federal Institute for Drugs and Medical Devices according to the Coronavirus Test Ordinance (TestV)
95.51% Sensitivity · 99.72% Specificity
With integrated buffer solution
Longsee Professional Rapid Antigen Tests: A Comprehensive Guide
Longsee 2019-nCoV Ag Rapid Test Kit (Immuno-chromatography)
Instructions for use
[INTENDED USE]
The rapid detection kit in this package is designed for the qualitative determination of SARS-CoV-2 nucleocapsid antigen in nasal or throat swab specimens from individuals suspected to have COVID-19 infection. The kit is intended for rapid diagnosis of SARS-CoV2 infections.
For professional use only. For in vitro diagnostic use only.
[SUMMARY]
The novel coronaviruses belong to the genus β. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Patients infected with the novel coronavirus are the main source of infection; asymptomatic infected persons can also be a source of infection. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations are fever, fatigue, and dry cough. In some cases, nasal congestion, runny nose, sore throat, myalgia, and diarrhea can also be observed.
[PRINCIPLE OF THE TEST]
This kit uses immuno-chromatography technology to detect the presence or absence of 2019-nCoV nucleocapsid proteins in swab specimens from patients with signs and symptoms of infection, where there is suspicion of 2019-nCoV by the double antibody sandwich method. When the concentration of 2019-nCoV antigens in specimens is higher or equal to the minimum detection limit, these antigens react separately with corresponding antibodies to form complexes, and the 2019-nCoV antibodies are coated in the detection area (T). These antigens are captured and a red reaction line is formed. The result is considered positive. Otherwise, the result formed in T without a red line is considered negative. Under normal test conditions, the quality control area (C) should be colored to indicate that the test is valid.;
[COMPONENTS]
| Component | 1T/kit | 5T/kit | 25T/kit | 50T/kit | |
| 1 | Test cartridge | 1 pc | 5 pcs | 25 pcs | 50 pcs |
| 2 | Single swab | 1 pc | 5 pcs | 25 pcs | 50 pcs |
| 3 | Extraction tube | 1 pc | 5 pcs | 25 pcs | 50 pcs |
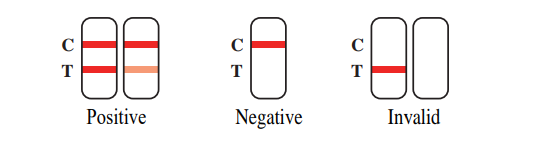
[RESULT EVALUATION]
To read the test results, you just need to look at the observation window.
1. Positive: If two color bands are visible at the position of the quality control line (C-line) and the test line (T-line) in the result window, the test result is positive. The test result indicates the presence of 2019-nCoV antigens in the sample.
2. Negative: If a color band appears at the position of the quality control line (C-line) and no color band appears at the detection line (T-line) in the observation window, the test result is negative. The test result indicates that the sample is negative for 2019-nCoV antigens or the concentration is below the detection limit of the set.
3. Invalid: If no band appears at the position of the quality control line (C-line) and the test line (T-line) in the observation window, the test result is INVALID. The sample should be re-taken and the test repeated.
[LIMITATIONS]
1. The Longsee Rapid Detection Kit is intended for professional use only and for in vitro diagnostics.
2. This kit is designed to detect the presence of SARS-CoV-2 nucleocapsid antigen in nasal or throat swab specimens from individuals with suspected COVID-19 infection. It is not intended to accurately determine the content of antigens in samples.
3. The accuracy of the results can be affected by improper sample collection and storage.
4. Test results should not be used as the sole basis for clinical diagnosis and treatment. A comprehensive consideration of laboratory examination and treatment response should be taken into account.
5. Negative results must be combined with other test results to make a comprehensive assessment. It is recommended to use nucleic acid tests or methods for virus culture identification for verification and confirmation.
6. False-negative results can be caused by improper sample collection, transport, and processing, as well as low virus titers in the sample. Viral gene mutations can also cause changes in antigenic determinants and result in false-negative results.
You can download the Longsee Rapid Detection Kit guide here.
General FAQ
How does Odem ensure high quality at such a fair price?
The Better AG, founded in 2006 in Switzerland, has become the main supplier for thousands of companies in recent years.
The strategy:
• Bulk purchasing
• Close quality control of the goods
• Passing on the purchasing advantages to our customers
• By offering the possibility to receive free samples, our customers are not taking any risks.
What is the money-back guarantee?
If you are not satisfied with your goods, you can return them within 14 days of purchase and receive a full refund.
What payment options are available?
We offer a convenient payment by invoice after receipt of the goods to a German bank account.
Has the goods already been cleared through customs?
Yes, the goods have already been cleared through customs and will be delivered to you from our German warehouse. You will not incur any additional costs.
Product sheet
Login